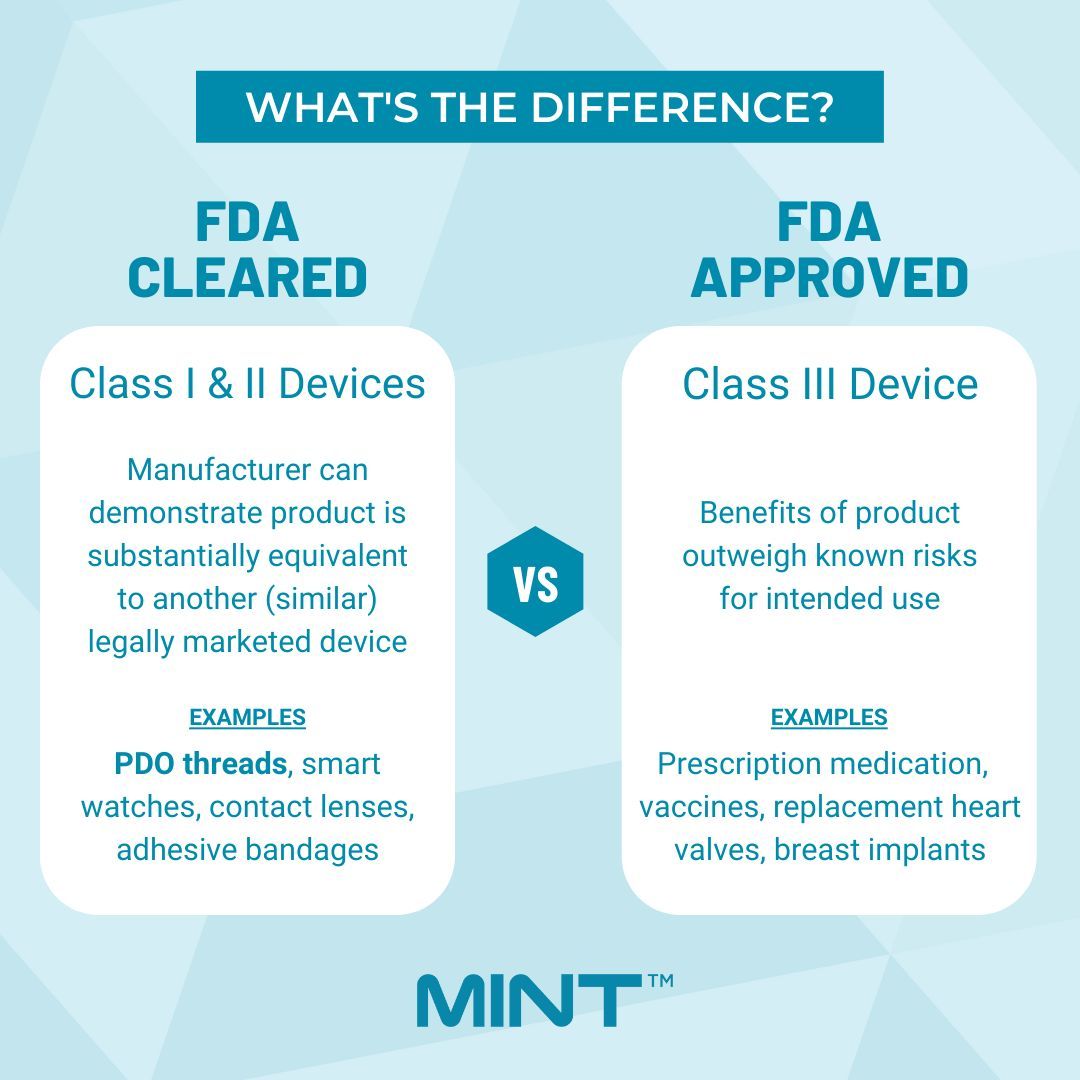
—FDA Cleared vs FDA Approved—
???????????? ????????????????????????????Lower-risk products classified as Class I (ex adhesive bandages, wheelchairs, tongue depressors) and Class II (ex ???????????? ????????????????????????????, contact lenses, pregnancy test kits) need to undergo a 510(k) submission, which the FDA then reviews and provides clearance.???????????? ????????????????????????????????Premarket approval (PMA) is the most stringent type of device marketing application required by the FDA. Devices classified as Class III (ex pacemakers, defibrillators, high-frequency ventilators) require FDA approval prior to marketing due to the level of risk associated with them.FDA Cleared and FDA approved medical devices undergo entirely different processes. If companies do not specify their PDO threads are FDA cleared or market their PDO threads as FDA approved, be very cautious!⚠️MINT™ PDO is a Class II medical device and the only PDO threads with TWO FDA clearances. It is also backed by more than 6 years of published studies for its safety and efficacy so that we can offer the best quality of threads out there on the market.
Premier Skin Clinic is a proud provider of MINT Threads.
Schedule an consultation with us today! (970) 221-1285

